Research Themes
Pathogen-agnostic effects of BCG vaccination
Bacille Calmette Guerin (BCG; a live vaccine containing attenuated Mycobacterium bovis), the vaccine against Tuberculosis (TB), is one of the oldest vaccines still given and one of the most administered in vaccine history. The benefits of BCG vaccination extend far beyond offering protection against TB and have been found to reduce risk for serious infections resulting in deaths or hospitalizations from unrelated infections; called non-specific or pathogen-agnostic effects of the BCG vaccine. These pathogen-agnostic effects are especially powerful when BCG is given to newborns, who are at exceptionally high risk to die of infectious diseases; BCG-vaccinated newborns in low resource settings have half the risk to die compared to unvaccinated newborns. These effects involve the way that the BCG vaccine alters innate immunity. BCG induces a process called “trained immunity”, which epigenetically reprograms stem cells in the bone marrow to give rise to monocytes that are “trained” to mount a more robust inflammatory response to unrelated stimuli. In newborns, our team discovered that BCG also induces a process called “emergency granulopoiesis (EG)”, or a rapid production of mature neutrophils, which are then able to rapidly clear invading microbes. EG explains the beneficial pathogen-agnostic effects of BCG vaccination in newborns. The cytokine Granulocyte-Colony Stimulating Factor (G-CSF) initiates EG.
BCG Insitu: Untangling the link between skin reactions to BCG vaccination and improved newborn health outcomes

We have not identified how BCG initiates the production of G-CSF: which molecular sensors are used, and which cells mount this initial response. Also, why BCG induces G-CSF and EG in newborns, but not in adults is also a mystery. Given that BCG is given into the skin as an intradermal injection, the answer likely lies in this tissue. See our Vacancies if you would like to work on this!
Fuel for Survival: metabolic interventions to prevent neonatal sepsis
Of all children that die in the first 5 years of life, half are newborns. Sepsis, a host response to systemic microbial infection, is a leading cause. While vaccines have eliminated a substantial burden of severe infection among older children, most are not given to neonates, and the time required to mount an adaptive immune make them ineffective for neonatal infection prevention. With this, therapies that broadly enhance host resilience to infection, that could work quickly while being feasible to implement in low-resource settings are most likely to make a difference.
Fuelling granulopoesis
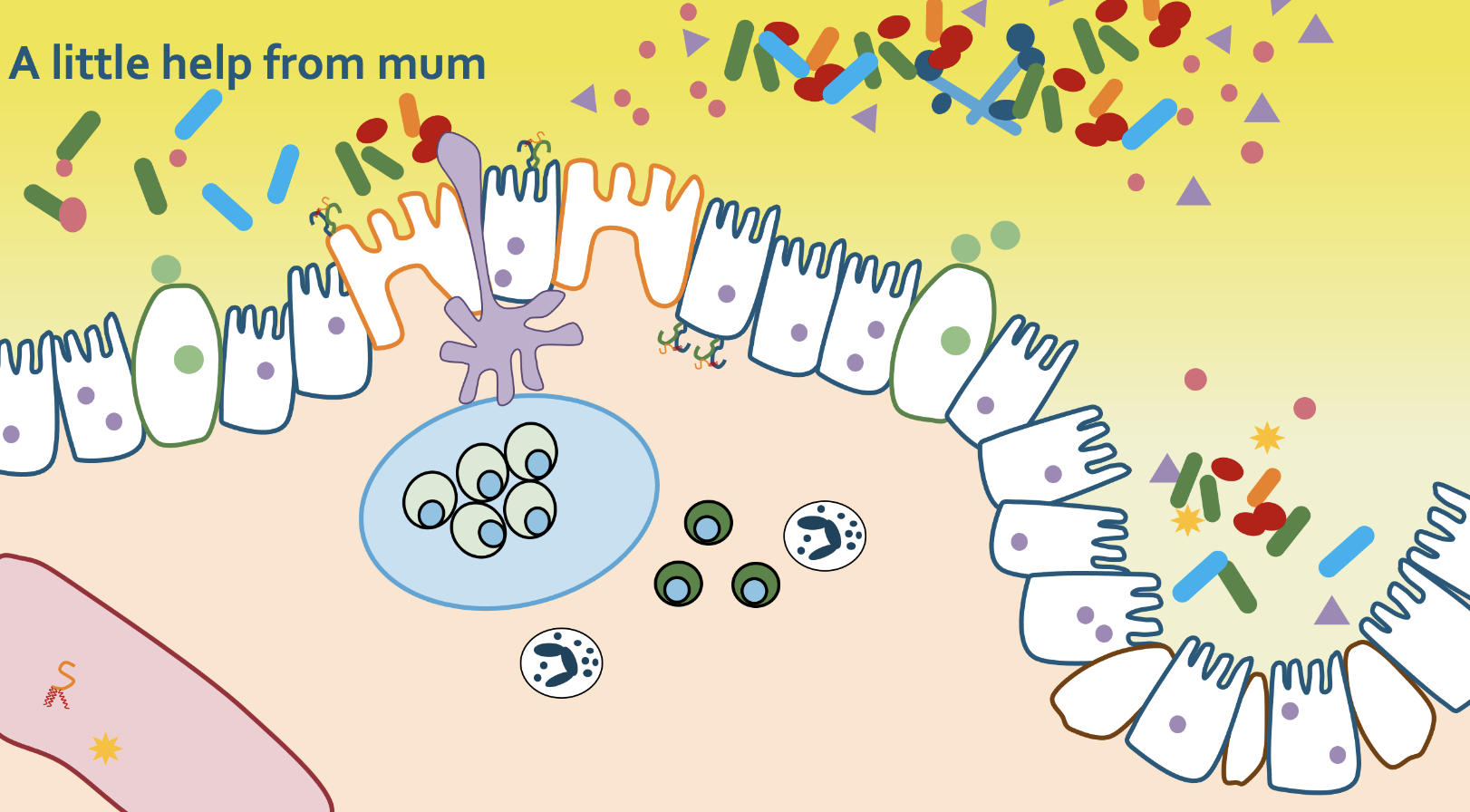 Given that not all newborns benefit from BCG vaccination, we hypothesize that this is because immune boosters like BCG need “fuel” to work. Robust data from human studies had found that newborns that are not fed colostrum, the first breast milk, within an hour of birth are more likely to die from infectious disease. Research by our colleage Valerie Verhasselt also demonstrated the critical role of colostrum in newborn immune health. Given the strong relationship between nutrition and immune responses, we are now investigating how there critical first feeds impact on neonatal sepsis and the response to BCG. See our Vacancies if you would like to work on this!
Given that not all newborns benefit from BCG vaccination, we hypothesize that this is because immune boosters like BCG need “fuel” to work. Robust data from human studies had found that newborns that are not fed colostrum, the first breast milk, within an hour of birth are more likely to die from infectious disease. Research by our colleage Valerie Verhasselt also demonstrated the critical role of colostrum in newborn immune health. Given the strong relationship between nutrition and immune responses, we are now investigating how there critical first feeds impact on neonatal sepsis and the response to BCG. See our Vacancies if you would like to work on this!
The microbiome in early life
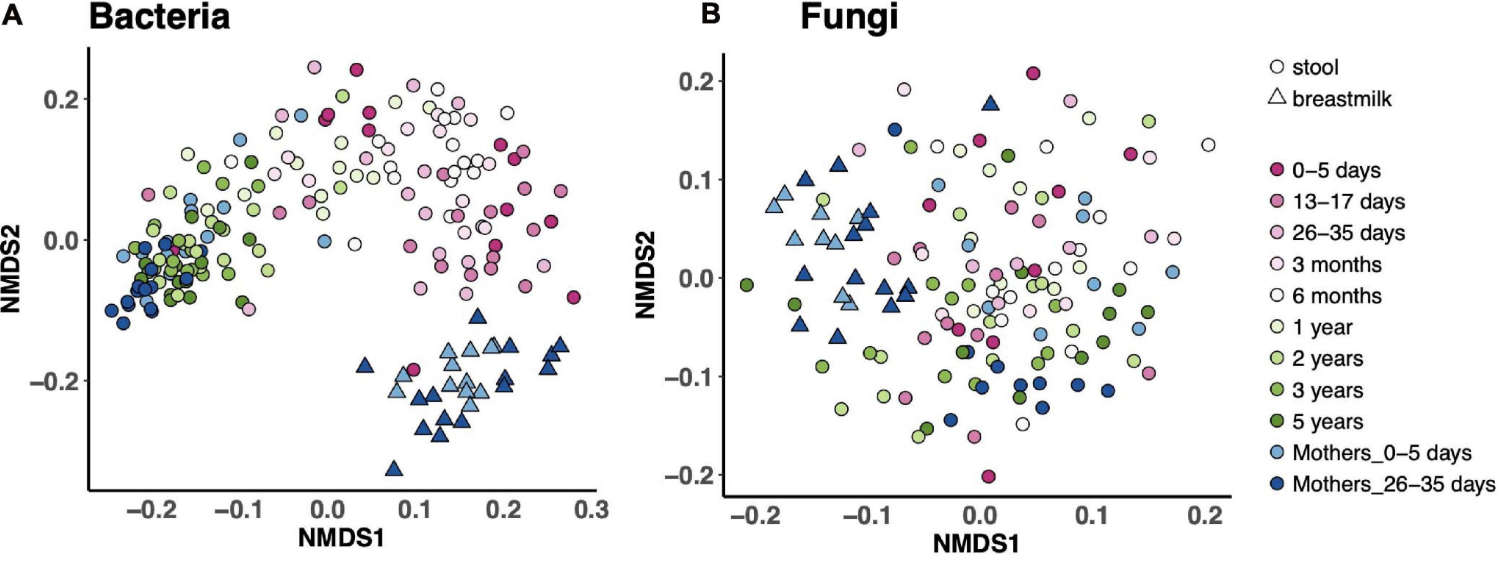
The gut microbiome is a like another organ in our bodies, with microbial communities performing much of the work to keep us healthy. The microbiome likely plays a critical role in establishing immune resilience, and may in part shape your responses to infections and vaccinations. We hypothesized that early life is an especially critial window of opportunity to optimize microbial community assembly for long-term health benefits. Indeed, some of our collabrative work found that Canadian infants that went on to develop atopy at 3 years of age had lower levels of butyrate-producing bacteria in their stool at 3 months, but not at one year of age. Our work on the microbiome involves finding associations between the microbiome and immune health (including responses to BCG vaccination), and to determine whether probiotics given early in life can improve newborn infectious outcomes.
Ontogeny of the newborn microbiome in the first week of life
We work with the Expanded Program of Immunization Consortium; EPIC, an NIH-funded study enrolling over 700 newborn babies in the Gambia and Papue New Guinea. By measuring the gut microbiome of these babies during the first week of life using shallow-shotgun metagenomics, we will be able to determine the main drivers of microbial ontogeny in the newborn period, how host factors such as newborn feeding impact on this development, and how the microbiome in turn may impact responses to BCG vaccination or predict onset of neonatal sepsis.
Maternal Probiotics for neonatal colonization
 Probiotics have a long and complex history. However, one of the most promising studies showing possible benefits to reduce newborn infection showed that giving newborns L. plantarum at birth dramatically reduced the risk for sepsis. However, giving probiotics directly to newborns is not always feasible. Together with our colleages from the KHRC, we are testing the feasibility and safety of providing L. plantarum directly to mothers during the 3rd trimester of pregnancy in Rural Ghana. Metagenomic analysis of maternal and neonatal stool samples will also reveal whether the introduction of this nomadic microbe will alter the composition or functional capacity of their microbiomes.
Probiotics have a long and complex history. However, one of the most promising studies showing possible benefits to reduce newborn infection showed that giving newborns L. plantarum at birth dramatically reduced the risk for sepsis. However, giving probiotics directly to newborns is not always feasible. Together with our colleages from the KHRC, we are testing the feasibility and safety of providing L. plantarum directly to mothers during the 3rd trimester of pregnancy in Rural Ghana. Metagenomic analysis of maternal and neonatal stool samples will also reveal whether the introduction of this nomadic microbe will alter the composition or functional capacity of their microbiomes.